- Visibility 75 Views
- Downloads 9 Downloads
- DOI 10.18231/j.ijcap.2021.008
-
CrossMark
- Citation
Study of variations in the anterior half of the circle of Willis using magnetic resonance angiography
- Author Details:
-
Merlin Leena Rajan *
Introduction
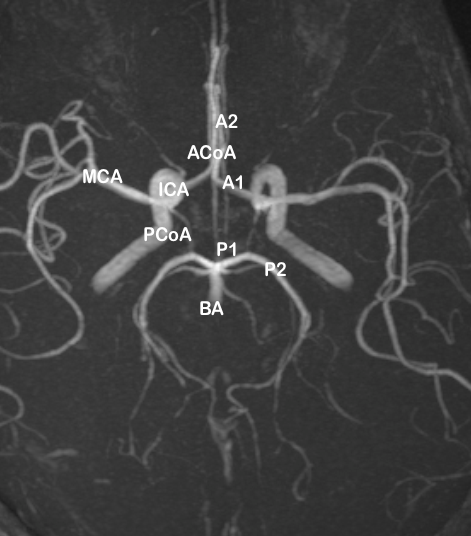
The circle of Willis is an arterial anastomosis at the base of the brain that unites the internal carotid and vertebro-basilar arteries.[1] Anteriorly, the anterior cerebral arteries, branches of internal carotid, are connected by anterior communicating artery, and posteriorly, the posterior cerebral artery, one on each side arising from basilar artery, is joined to the ipsilateral internal carotid by posterior communicating artery. Clinicians frequently divide the intracranial vasculature2 into an anterior circulation consisting of intradural internal carotid and its two terminal branches, namely anterior cerebral and middle cerebral arteries, and the anterior communicating artery, and a posterior circulation comprising of posterior communicating arteries, vertebro-basilar trunk and the terminal branches of basilar artery, the two posterior cerebral arteries.
In the anterior part, the anterior cerebral artery (ACA) arises from the medial aspect of the internal carotid artery bifurcation, below the anterior perforated substance, at the medial end of the stem of the lateral sulcus. It courses antero-medially towards the inter-hemispheric fissure, separated by the falx cerebri, above the optic chiasma or optic nerve. At the level of the inter-hemispheric fissure, it is joined to the contralateral anterior cerebral artery via, the short transverse anterior communicating artery (ACoA) in the supra-chiasmatic cistern.
Surgical nomenclature[2], [3], [4] divides the anterior cerebral artery (ACA) into three parts:
A1 (horizontal) segment extending from the internal carotid artery bifurcation to the anterior communicating artery.
A2 (vertical) segment extending from the junction with anterior communicating artery to the origin of callosomarginal artery (junction of the rostrum and genu of the corpus callosum).
A3 (callosal) segment distal to the origin of callosomarginal artery (extending around the genu until the artery turns sharply posteriorly, also called pericallosal artery).
To understand the variants in the circle of Willis observed during cerebral angiography, an understanding of its development is essential. The embryological development of the cranial arterial vasculature in its adult form evolves in early foetal life through a number of simultaneous/ overlapping steps. The third aortic arch is the precursor[5], [6] of the primitive internal carotid artery, which gives off cranial and caudal divisions that form the anterior cerebral and posterior communicating arteries respectively (also giving off anterior choroidal and middle cerebral arteries).
There are considerable individual variations in the pattern and calibre of vessels that make up the circulus arteriosus. The cerebral and communicating arteries may be individually absent, variably hypoplastic, double, or even triple. Widespread clinical interest in the arterial circle is due to its propensity for variations relevant for neurosurgical, vascular and radiological interventional procedures especially with regard to cerebrovascular accidents.
Materials and Methods
The study design is an observational descriptive study over a period of 18 months conducted by the department of Anatomy in coordination with department of Radio-Diagnosis at Pushpagiri Institute of Medical Sciences & Research Centre, Tiruvalla. The investigative modality for the study was magnetic resonance angiography (MRA) of the brain. Cerebral imaging was performed on a 1.5T MRI machine including axial T1, T2, and FLAIR (fluid attenuation inversion recovery) weighted sequences. Circle of Willis MRA was performed using three-dimensional (3D) time-off flight (TOF) sequence. The original MRA sequence for each patient is retrieved from an electronic database (DXMMR). The 215 subjects for the MRA study were taken up from those availing this investigation as part of their clinical workup or follow-up at the institute, on in-patient or outpatient basis. All MRAs of brain conducted in the institution on adults of both sexes in the age group between 18 and 90 years were included in the study. Those excluded were images with evidence of Neurosurgical interventions (AVM repair, shunting), known or diagnosed cases of vasculopathies such as arteritis, and known craniofacial anomalies and genetic disorders.
Results
In the present study of angiograms of 215 consecutive subjects who underwent MR angiography at our institute, the number of morphologically complete circle of Willis was 135 (62.79%). The textbook type Circle of Willis with complete anterior and posterior circulations was observed in 86 images (40% of total images); this formed 63.7% of the complete arterial circles. Incomplete arterial circles comprised 80 of the total 215 images (37.21%). The anterior circulation was functionally complete in 200 images, of which 86 had textbook configuration.
A1 segment of Anterior cerebral artery (ACA)
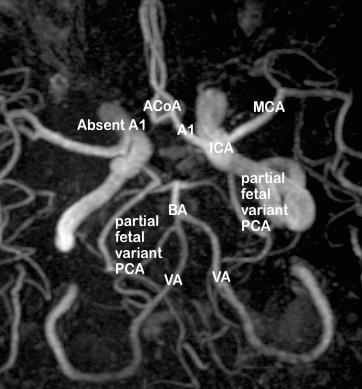
The variations observed in the A1 segments of right and left anterior cerebral arteries have been compiled as follows:
|
Type of variation |
Right A1 |
Left A1 |
Total |
Incidence |
|
Absent |
9 (4.18%) |
3 (1.4%) |
12 |
2.79% |
|
Duplicated |
2 (0.93%) |
3 (1.4%) |
5 |
1.16% |
|
Hyperplastic |
2 (0.93%) |
- |
2 |
0.46% |
|
Hypoplastic |
31 (14.42%) |
17 (7.9%) |
48 |
11.16% |
|
Tortuous |
2 (0.93%) |
4 (1.86%) |
6 |
1.39% |
|
Normal |
169 (78.6%) |
188 (87.44%) |
357 |
83.02% |
|
Total |
215 (100%) |
215 (100%) |
430 |
100% |
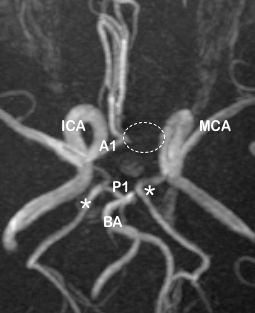
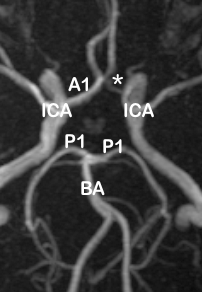
In the anterior circulation, the right A1 segment was normal in 169 images (78.6%), and the left A1 was normal in 188 images (87.44%). The most frequent variation of A1 segment was hypoplasia, which was seen in 31 cases (14.42%) on the right side and 17 (7.9%) on the left ([Table 1]).
Anterior communicating artery (ACoA)
The ACoA was normal in 185 images (86.04%), the most common variant being the fused ACA variety (9.3%) as depicted in [Table 2].
|
Type of variation |
Number of images |
Incidence (%) |
|
Absent |
1 |
0.47 |
|
Duplication |
1 |
0.47 |
|
Fenestrated |
8 |
3.72 |
|
Fused ACA |
20 |
9.3 |
|
Normal |
185 |
86.04 |
|
Total |
215 |
100 |
ACoA appeared fenestrated in eight cases, and was duplicated in one MRA. The artery was totally absent in one image.
Both duplication of ACoA and its absence were seen associated with normal pattern left A1 segment in one image each. At the same time, in five cases, fenestration of ACoA was associated with the normal left A1 segment, which was seen in 166 images and found to be statistically significant (Fisher’s two tailed p= 0.0421).
Duplication of left A1 seen in three cases was associated with fenestration of ACoA in two MRA image, while a normal left A1 (seen in 188 cases) was associated with fenestrated ACoA in five MRA, which was statistically significant (Fisher’s two tailed p=0.0034).

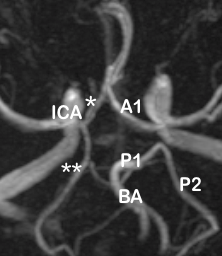
Discussion
Over the last three hundred years, many studies and case reports have been published regarding the cerebral arterial circle or circle of Willis (CoW). The early studies were mostly cadaveric dissection studies, the largest of those being on 994 brain specimens by Riggs and Rupp[7] in 1963. The introduction of radiological imaging for visualizing the circle of Willis came during the 1980s, initially using conventional cerebral angiography, and later by CT angiography and MR angiography.
Jesic et al.,[8] studied the variations of the anterior part of the CoW in 1000 subjects of the Serbian population using MRA, and found the classical pattern in 643 (64.3%) cases. Anterior CoW variations were seen in 113 (11.3%), and 244 (24.4%) had posterior CoW anomalies
The ACoA was studied in 100 formalin fixed brains by Kardile et al.[9] in 2013, with 38 variations reported. The most common variation was duplication (10), with absence of ACoA (8) and fusion (3).
The MR angiographic images in the present study were divided into anterior and posterior parts ([Figure 7]), and sub-classified into complete and incomplete variants.
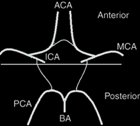
The distribution of the types of variants in the anterior part of the circle of Willis, as observed in the present study, is tabulated in [Table 3].
|
Type |
A |
B |
C |
D |
E |
F |
Total |
|
MR Angiograms |
130 (60.46%) |
1 (0.46%) |
5 (2.33%) |
23 (10.7%) |
1 (0.46%) |
55 (25.58%) |
215 |
In the present study, the observed patterns of variations in the anterior part of the circle of Willis were classified into six patterns ([Figure 8]) as follows:
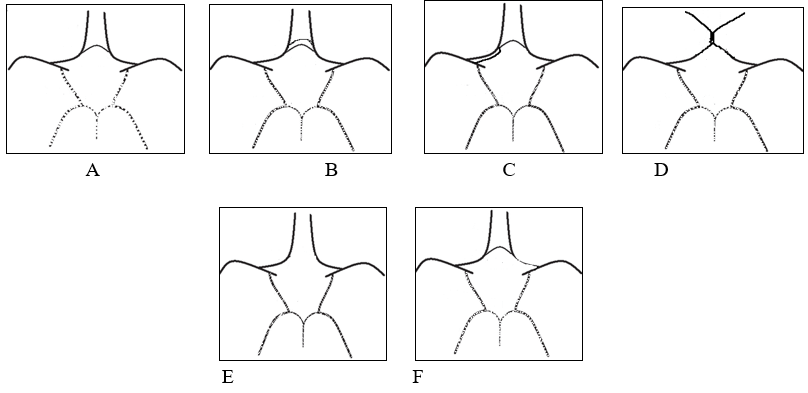
In the above classification of the MRA images in the present study, types A-D is considered as complete, and types E-F are considered as incomplete.
In the anterior part of the circle of Willis, the normal complete pattern (type A) was observed in 60.46% of cases. However, the highest incidence of variation was seen with absence or hypoplasia (type F) of unilateral A1 segment (right and left both inclusive) in 25.88% of MRA, and this accounts for a higher incidence of incomplete anterior CoW. Fused (short segment) ACA (type D) was observed to rank as the second commonest variation with a 10.7% incidence but as the arterial pathway was essentially intact, it was categorized as complete.
Embryological and clinical correlations
In order to explain the occurrence of the variations, it is necessary to review the embryogenesis of these vessels. The development[5], [6], [10], [11], [12], [13] of the cerebral arteries begins at approximately five weeks of gestation, and the primitive ICA (which arises from the dorsal aorta except the first segment which comes from the third aortic arch) is vital to the normal development of the brain and surrounding structures, being the embryologic precursor to the ACA, PCoA and MCA. In the 04 mm embryo, the carotid system supplies the forebrain and contributes to perfusion of the hindbrain through the primitive segmental arteries. In the 31-day embryo, all blood supply to the cerebrum comes from the ICA.
According to Padget,[5], [6], [10] the development of anterior cerebral circle is due to the initial development of numerous arteries from the primitive ICA to supply the forebrain, followed by the regression of certain arterial segments in utero and in adult life. In foetal life, there are three ACAs connected by an anterior communicating plexus; the third (or middle ACA) and the communicating plexus should normally regress, and failure to do so results in anatomical variants such as duplication of ACA, or duplication of ACoA. Conversely, the vessel segments that should persist may abnormally regress, causing hypoplasia/ absent ACoA or hypoplasia/ absent ACA. In the event of a cerebrovascular accident (such as stroke) affecting the anterior circulation, hypoplasia or absence results in significantly reduced collateral supply, and hence leads to an increased risk for larger area of cerebral ischaemia.
Abbreviations
CoW – Circle of Willis; ICA – Internal carotid artery; ACA – Anterior cerebral artery; ACoA – Anterior communicating artery; MCA – Middle cerebral artery; BA – Basilar artery; PCA – Posterior cerebral artery; PCoA – Posterior communicating artery; MRA – Magnetic Resonance Angiography.
Conflict of Interest
None.
References
- S Standring, AR Crossman. Neuroanatomy: Vascular supply and drainage of the brain. Gray’s anatomy 2008. [Google Scholar]
- P Morris. Part 2 Anatomy. Practical Neuroangiography 1997. [Google Scholar]
- AG Osborn. . Osborn’s Brain- Imaging, Pathology and Anatomy 2013. [Google Scholar]
- P Butler, AWM Mitchell, JC Healy, KM Hogarth, J Jarosz, P Butler. Applied Radiological Anatomy. Central Nervous System: The Skull and Brain 2012. [Google Scholar]
- DH Padget, WE Dandy. Intracranial arterial aneurysms. The circle of Willis. Its embryology and anatomy 1948. [Google Scholar]
- DH Padget. The development of the cranial arteries in the human embryo. Contrib Embryol 1948. [Google Scholar]
- HE Riggs. Variation in Form of Circle of Willis. Arch Neurol 1963. [Google Scholar] [Crossref]
- A Jesic, S Torbica, S Maric, S Popovic, D Kozic. Anatomic variations of the anterior portion of the circle of Willis: an MR angiography study. Curr Top Neurol Psychiat Relat Discip 2011. [Google Scholar]
- PB Kardile, JM Ughade, SV Pandit, MN Ughade. Anatomical variations of anterior communicating artery. J Clin Diagn Res 2013. [Google Scholar]
- DH Padget. Designation of the embryonic intersegmental arteries in reference to the vertebral artery and subclavian stem. Anatomical Rec 1954. [Google Scholar] [Crossref]
- SK Battacharji, EC Hutchinson, AJ Mccall. The circle of Willis- the incidence of developmental anomalies in normal and infarcted brains. Brain 1967. [Google Scholar]
- DB Moffat. The embryology of the arteries of the brain. Ann R Coll Surg Engl 1962. [Google Scholar]
- D B Moffat. The development of posterior cerebral artery. Journal Anat Physiol . [Google Scholar]
