- Visibility 90 Views
- Downloads 15 Downloads
- DOI 10.18231/j.ijcap.2020.067
-
CrossMark
- Citation
Histological aspect of left atrial appendage (LAA) with special stain-masson’s trichome
- Author Details:
-
Garima Pardhi
-
Savita Gadekar *
Introduction
The left atrium possess a venous component, a vestibule and an appendage and shares the septum. The Left Atrial Appendage (LAA) is characteristically a small finger like extension in human hearts and has crenations over all lobes that are potential sites for disposition of thrombus.[2]
For decades anatomy text books tended to focus on the ventricles because of their important role as pumping chambers. The two atria have long been looked at as collecting chambers, or even just conduits for blood to get through to the ventricles, hence attracted less interest for studying them.
The structure of the heart undergoes constant adaptation to physiologic changes in the organism. These changes are varied and take place from the time of embryonic development to senescence. The difference between physiological and pathological adaptive mechanisms is sometimes subtle. Therefore, it is important to understand that the structure of the heart is modified by a continuum of evolving adaptation of the organ to the needs of the organism. In many instances, pathologic changes affecting the heart have a structural substrate, whether they are due to genetic or acquired processes. Like any other organ, the heart has cells that contribute to the formation of the stroma (the connective tissue scaffold of the organ) and cells that form the parenchyma (the physiological-working-tissue of the organ, in the case of the heart these are the myocytes). The basic cellular unit of the parenchyma of the cardiac muscle is the cardiac myocyte.[3]
Actually, the LAA is a remnant of the original embryonic left atrium formed during the third week of gestation. The LAA lies within the pericardium in close contact with the free wall of the left ventricle. It is therefore likely that blood flow, in and out of the LAA, depends to a significant degree on a properly functioning left ventricle. The LAA empties into the left atrium through an orifice located between the left upper pulmonary vein and the left ventricle.[4]
The trabecular LAA is a remnant of the original embryonic left atrium that develops during the third week of gestation. The main smooth-walled left atrial cavity develops later and is formed from an outgrowth of the pulmonary veins. The function of the LAA is unknown. In studies performed more than 50 years ago, it was speculated that the atrial appendages fill the space that is created within the pericardial sac during ventricular systole as the ventricles eject blood and decrease in size. The appendages passively fill during ventricular systole and then passively empty during ventricular diastole.[5]
Atrial cardiocytes of the mammalian heart contain granules which are morphologically similar to those in peptide secreting endocrine cells. These secretory granules were discovered by Kisch (1956), a finding confirmed by several investigators (Posche, 1957; Bompiani et al., 1959). The granularity of the atria can be altered experimentally by salt and water loading or deprivation (De Bold, 1979) and by procedures which cause perturbation of salt and water balance; these include adrenal regeneration hypertension (Martinez, 1966) and adrenalectomy. These granules are known as Atrial Natriuretic factor (ANF).[6]
LAA is a major endocrine organ and is the main producer of ANP (atrial natriuretic peptide) in the human heart. The ANP concentration is 40 times higher in the LAA walls than in the rest of the atrial free wall and in the ventricles. A study of patients having undergone the maze procedure and associated LAA removal found a significantly lower ANP secretion and a commensurate increase in salt and water retention. Whether this could eventually lead to hypertension is not known.[4]
So, analysis of ANF has shown that LAA contain about 30% of all cardiac ANF. The cardiocytes of LAA contain the greatest density of ANF granules found in Left Atrium and it is therefore of interest to carry out further studies on these granules.[1]
Aims and Objectives
Looking to the great clinical significance that is given to the morphology of LAA & its predilection as a site for thrombus formation, we’ve conducted a detailed study histologically of LAA.
To investigate LAA and study the arrangement of muscle bundles of LAA histologically (with a special stain-Masson’trichome) and these findings were co-related with that described in the literature.
The specimens were photographed and photomicrography of appropriate histological sections was carried out.
These findings were correlated with those cited in the literature. An attempt was made to draw a correlation of these findings with the functions of LAA.
These inturn help in understanding on its clinical significance and would be of help manage these patients.
Materials and Methods
The materials for the present study were collected from the Department of Anatomy & Forensic medicine of Sri Aurobindo Medical College and Post Graduate Institute, Indore. Randomly five hearts were selected for histological purposes of Left Atrial Appendage (LAA) which included the following:
For histology: slides of LAA from different sites (apex, inferior margin and atrio-auricular junction) were prepared by routine Histological Techniques and stained.
Histology
Small pieces (2.0cm X 0.5cm) of LAA were taken from different sites (apex, inferior margin and atrio-auricular junction). The tissues were fixed in 10% formalin, and were processed for routine histological procedures – like dehydration, clearing and after wax embedding, blocks were prepared. Sections 5-6micron thick were cut and stained with the following stains:[7], [8]
H & E
Method for staining are-
Sections were dewaxed wit xylene and the sections were taken to water (with descending grades of alcohol).
Stain in haematoxylin, in a jar for 5-10minutes.
Wash well in running tap water for 2-3mnutes.
Remove excess stain by decolorizing (differentiating) in 0.5-1% hydrochloric acid in 70% alcohol for few seconds.
Wash in alkaline running tap water for 5minutes.
Stain in 1% aqueous eosin for 1minutes..
Wash surplus stain in water.
Dehydrate in alcohol, clear in xylene.
Mount in D.P.X. or Canada balsam.
Masson’s trichome
Method for staining are- 1. Dewax sections and hydrate to water.
Transfer to stain in weigert’s iron haematoxylin for 6 minutes.
Differentiate in acid-alcohol.
Wash in running tap water.
Stain with ponceau acid fuchsin for 2-5 minutes.
Rinse in tap water.
In 1% phosphomolybdic acid for 5-10 minutes.
Stain for 2 minutes in light green solution.
Differentiate in water.
Dehydrate through increasing strengths of alcohol (70%, 90%, absolute.
Clear and mount in D P X 7.
Summary and Conclusion
This study of its histological features is an attempt to establish the cause of various pathologies related to arrythmias.
The histological features of all the three regions of LAA studied conform to the classical description of all these layers of the atrial wall, with a few exceptions: i) some special category of cells were seen within the connective tissue of the endocardium; ii) sub-endocardial tissue showed stratification along with a number of elastic lamellae; iii) thick cardiomyocytes showed the presence of fine granules in the peri-nuclear zone; iv) at places the epicardium was lined by cuboidal to columnar cells.
The present findings will be of great help in the interpretation of Trans-Esophageal-Echocardiography (TEE) and the understanding of the thrombo-embolic phenomenon.
Observations
The Left Atrial Appendage (LAA) is a very unique structure within the pericardial cavity, although small in size and having a variegated appearance, it is notorious for arrhythmias and thrombo-embolic phenomenon. This study was undertaken with the objective that certain features of histological anatomy may help in elucidating certain special features of LAA which can be correlated with its tendency for malfunctioning.
Histology
Histological features of the three regions (atrio-auricular junction, inferior margin and apex) studied showed almost identical features with only very slight variations that have been included in the observations described below. Due to the same reason, only selected photomicrographs (of special stain-Masson’s trichome) have been included. Following are the microscopic findings:
Epicardium
The outer layer of the heart is termed epicardium. There was a of thin layer of epicardium where deep to it greater amount of adipose tissue was present and the epicardium was thick if adipose tissue was scarce or absent. These features were seen in all the three sites studied namely atrio-auricular junction, inferior margin and apex.
Lining cells of epicardium in (Atrio-Auricular Junction) AAJ showed the presence of flattened cells which at places were cuboidal to columnar, whereas at some places, in sections of apex and inferior margin lining of cuboidal cells was seen.
On a deeper plane variable amount of adipose tissue was present. Similar findings were seen in sections taken from apex and inferior margin. In the sub-epicardial region many large blood vessels and nerves were present.
At places in sections taken from apex, in sub-epicardial region there was large amount of fat and it was covered by flattened cells.
Myocardium
Myocardium of AAJ in the auricular wall showed variable thickness. At the site of musculi pectinati it was quite thick, elsewhere it was so thin that endocardium and epicardium were almost touching one another separated by very small amount of connective tissue.
Size of the fibers was variable. Fibers were oriented in different directions: where the atrial wall was thin the fibers were mostly circularly arranged. At places, longitudinal fibers were sandwiched between transversely oriented fibers.
There was presence of large number of blood vessels of various sizes and types seen between the muscle fibers. They were present in sections of all the three sites studied.
Musculi pectinati were seen as projections in the auricular cavity being lined by endocardium. In musculi pectinati there was presence of longitudinal and transverse fibers. Similar findings were observed in sections taken from apex and inferior margin.
Presence of granules, which were of deep purple color, were observed in peri-nuclear area in some of the muscle fibers. Similar findings were there in sections taken from apex and inferior margin.
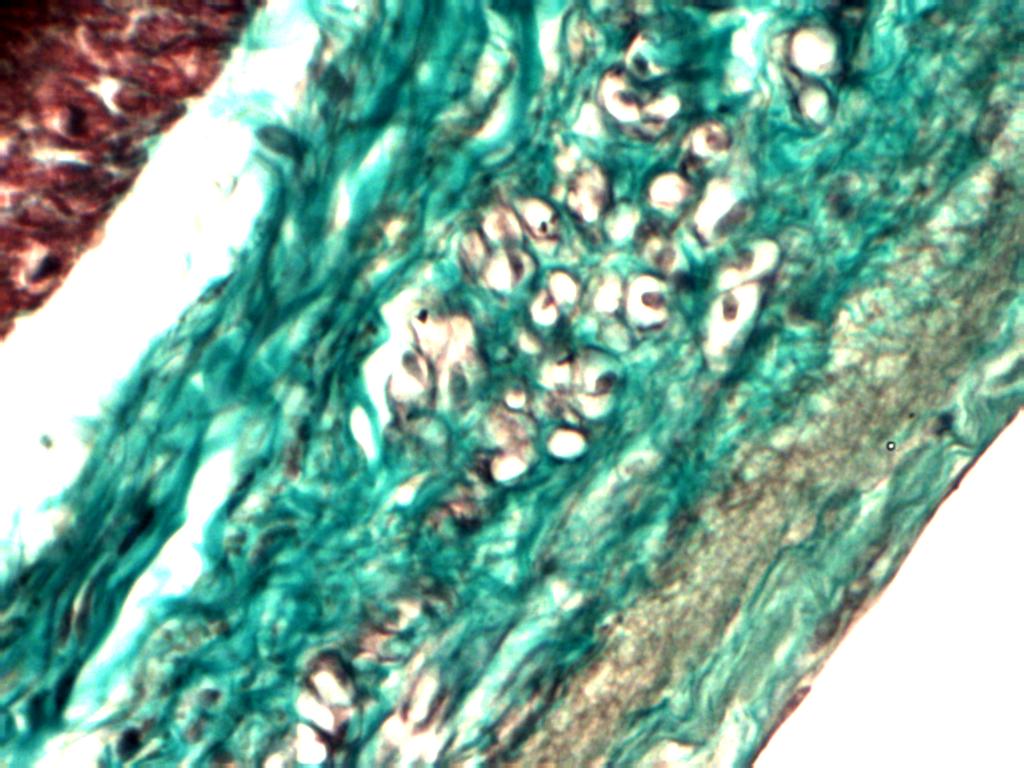
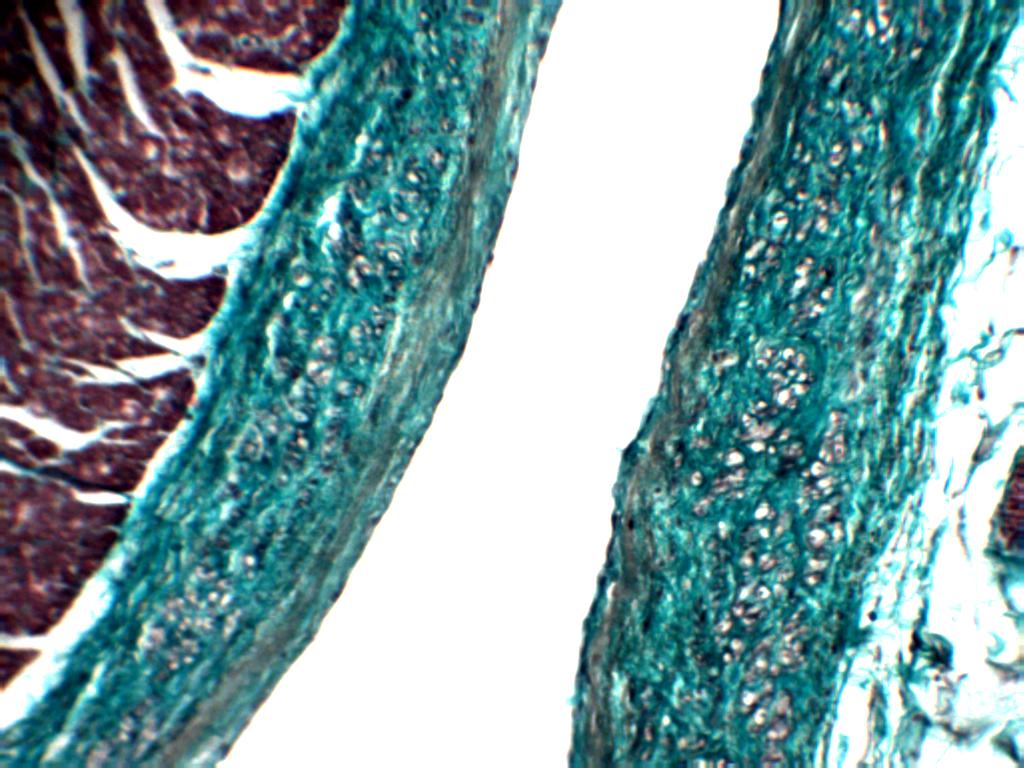

Endocardium
Some special cells were present in the endocardium, below the endothelial lining. These cells were present in groups and at distances, were darkly eosinophilic cytoplasm with small and centrally placed nucleus. These cells were not seen throughout the endocardium. Similar finding was seen in sections taken from apex and inferior margin.
In addition, in the deeper part of the endocardium at places rounded empty looking cells were present. These were also found in sections taken from apex and inferior margin. In one of the sections of inferior margin muscular artery was seen embedded in sub-endocardial region.
The atrial wall endocardium was smooth with flattened endothelial cells. Whereas, auricular wall showed projections. Where amount of loose connective tissue increased in the deeper part of endocardium, there were a number of undulations seen in the endocardial lining. At places sub-endocardial space with loose connective tissue was observed. There was absence of sub-endocardial space in some regions, so that the endocardium was almost adherent to myocardium.
In sections taken from apex, at places, musculi pectinati came close to each other thereby separating part of the auricular cavity.
Source of Funding
None.
Conflict of Interest
None.
References
- N M Al-Saady, O A Obel, A J Camm. Left atrial appendage: structure, function, and role in thromboembolism. Heart 1999. [Google Scholar]
- K. Wang, S Y Ho, D G Gibson, R H Anderson. Architecture of atrial musculature in humans.. Heart 1995. [Google Scholar]
- . Cardiac histology (I). e--heart.org. . [Google Scholar]
- H R Larsen. Left Atrial Appendage: Useless or Priceless? The Afib Report. . [Google Scholar]
- A J de Bold, J J Raymond, S A Bencosme. Atrial specific granule of the rat Heart Light Microscopic staining and Histochemical Reaction. J Histochem Cytochem 1978. [Google Scholar]
- G B M Lindop, E A Mallon, G Maclntyre. Atrial natriuretic peptide in the heart and pancreas. Histol Histopath 1986. [Google Scholar]
- H M Carleton, Rab Drury. . Histological technique for normal and pathological tissues and the identification of parasites 1957. [Google Scholar]
- C F A Culling. . Handbook of Histopathological and Histochemical Techniques 1975. [Google Scholar]
How to Cite This Article
Vancouver
Pardhi G, Gadekar S. Histological aspect of left atrial appendage (LAA) with special stain-masson’s trichome [Internet]. Indian J Clin Anat Physiol. 2025 [cited 2025 Sep 08];7(3):323-326. Available from: https://doi.org/10.18231/j.ijcap.2020.067
APA
Pardhi, G., Gadekar, S. (2025). Histological aspect of left atrial appendage (LAA) with special stain-masson’s trichome. Indian J Clin Anat Physiol, 7(3), 323-326. https://doi.org/10.18231/j.ijcap.2020.067
MLA
Pardhi, Garima, Gadekar, Savita. "Histological aspect of left atrial appendage (LAA) with special stain-masson’s trichome." Indian J Clin Anat Physiol, vol. 7, no. 3, 2025, pp. 323-326. https://doi.org/10.18231/j.ijcap.2020.067
Chicago
Pardhi, G., Gadekar, S.. "Histological aspect of left atrial appendage (LAA) with special stain-masson’s trichome." Indian J Clin Anat Physiol 7, no. 3 (2025): 323-326. https://doi.org/10.18231/j.ijcap.2020.067
